What Information About an Atom's Properties Can You Read From the Periodic Table?
Periodic Table of Elements
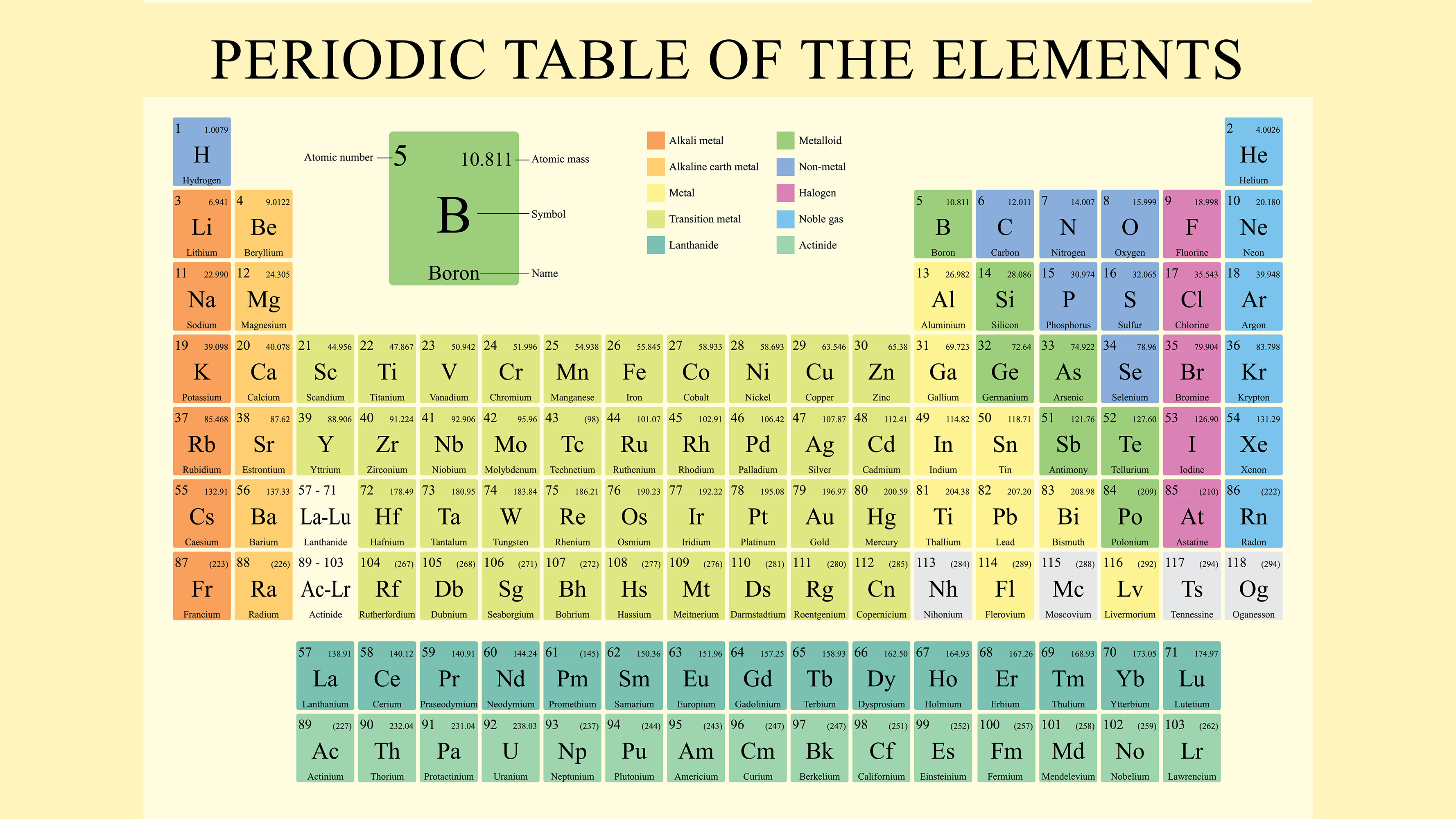
The periodic tabular array, besides called the periodic table of elements, is an organized arrangement of the 118 known chemical elements. The chemical elements are arranged from left to right and top to bottom in order of increasing diminutive number, or the number of protons in an cantlet'southward nucleus, which more often than not coincides with increasing atomic mass.
The horizontal rows on the periodic tabular array are called periods, where each flow number indicates the number of orbitals for the elements in that row, co-ordinate to Los Alamos National Laboratory. (Atoms take protons and neutrons in their nucleus, and surrounding that, they accept their electrons arranged in orbitals, where an atomic orbital is a math term that describes the location of an electron as well as its wave-like behavior.)
For instance, period 1 includes elements that have one atomic orbital where electrons spin; period 2 has two atomic orbitals, period iii has iii and so on up to period 7. The columns, or groups, on the periodic tabular array correspond the atomic elements that have the same number of valence electrons, or those electrons in the outermost orbital shell. As an case, elements in Group 8A (or VIIIA) all have a full set of eight electrons in the highest-energy orbital, according to chemist William Reusch, on his webpage at Michigan State Academy. Elements that occupy the same column on the periodic table (chosen a "group") have identical valence electron configurations and consequently bear in a similar fashion chemically. For example, all the group xviii elements are inert gases, meaning they don't react with any other elements.
Related: How are the elements grouped?
Who created the periodic table?
Dmitri Mendeleev, a Russian chemist and inventor, is considered the "father" of the periodic table, according to the Regal Club of Chemical science. In the 1860s, Mendeleev was a pop lecturer at a university in Saint petersburg, Russia. At the time, no mod organic chemistry textbooks in the Russian linguistic communication existed, so Mendeleev decided to write one. As he was working on that book, titled "Principles of Chemical science" (two volumes, 1868–1870), he simultaneously tackled the problem of the disordered elements, according to Khan Academy.

Putting the elements in whatsoever kind of guild would prove quite difficult. At the time, there were 63 known chemic elements, each with an atomic weight calculated using Avogadro'southward hypothesis, which states that equal volumes of gases, when kept at the same temperature and force per unit area, hold the same number of molecules.
Just two strategies existed at the time to categorize these elements: separating them into metals and nonmetals or grouping them by an element'southward number of valence electrons (or those electrons in the outermost shell). The first section of Mendeleev's book dealt with just viii of the known elements — carbon, hydrogen, oxygen, nitrogen, chlorine, fluorine, bromine and iodine — and those two strategies worked for those particular elements, according to Michael D. Gordin in his book "A Well-Ordered Matter: Dmitrii Mendeleev and the Shadow of the Periodic Table" (Princeton University Printing, Revised Edition 2018). Only they weren't enough to usefully sort the 55 additional chemical elements known at the time.
And then co-ordinate to the Majestic Society of Chemistry, Mendeleev wrote the properties of each chemical element on cards, and so he started ordering them by increasing atomic weight. This is when he noticed certain types of elements regularly actualization and noticed a correlation between atomic weight and chemical properties.
But the exact Eureka! moment that led Mendeleev to the sorting strategy that produced his complete periodic tabular array is shrouded in mystery. "Information technology is extremely difficult to reconstruct the procedure past which Mendeleev came to his periodic arrangement of elements in terms of their atomic weights," Gordin wrote of the full periodic table. "The trouble from the historian'southward perspective is that while Mendeleev kept almost every document and draft that crossed his easily after he believed he would get famous, he did non do and then before his formulation of the periodic law."
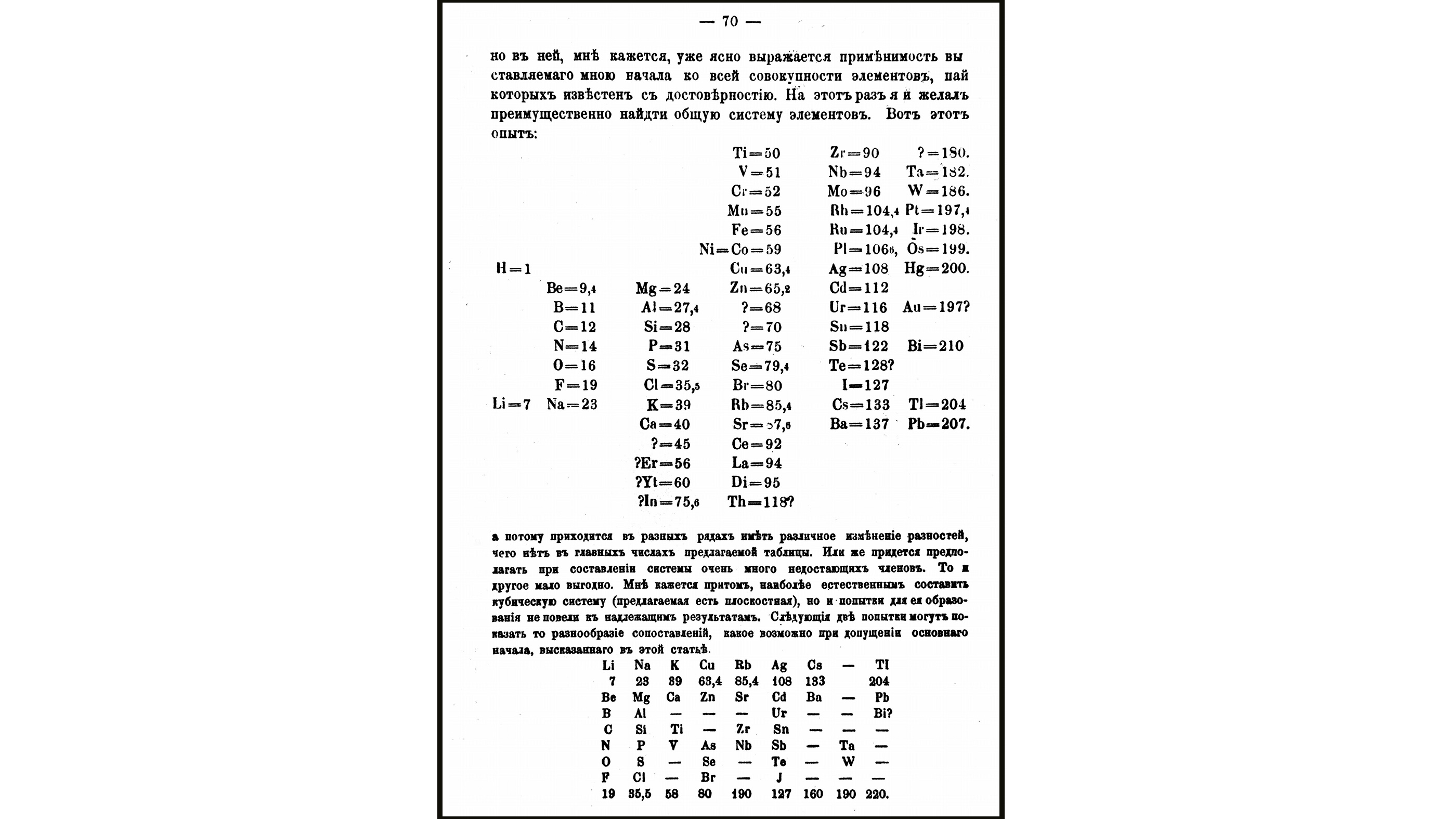
Gordin continued, "There are two basic means that Mendeleev could have moved from a recognition of the importance of atomic weight as a good classifying tool to a draft of a periodic system: either he wrote out the elements in order of atomic weight in rows and noticed periodic repetition or he assembled several 'natural groups' of elements, similar the halogens and the alkali metals, and noticed a pattern of increasing weight." Turns out, the only known statement from Mendeleev that was related to his method came in Apr 1869; he wrote that he "gathered the bodies with the lowest atomic weights and placed them past club of their increase in atomic weight," co-ordinate to Gordin's book.
Whatever his idea process, Mendeleev ultimately arranged the elements according to both atomic weight and valence electrons. Not only did he exit space for elements not yet discovered, but he predicted the properties of five of these elements and their compounds. In March 1869, he presented the findings to the Russian Chemical Society. Later that year, his new periodic system was published equally an abstract in the German language chemistry periodical Zeitschrift fϋr Chemie (Journal of Chemical science), according to the University of California, San Diego.
Reading the Periodic Tabular array
The periodic tabular array contains an enormous amount of information:
Atomic number: The number of protons in an atom'southward nucleus is referred to equally the diminutive number of that element. The number of protons defines what element it is and also determines the chemical behavior of the chemical element. For instance, carbon atoms always take six protons; hydrogen atoms ever have one; and oxygen atoms e'er have 8. Different versions of the same element, called isotopes, tin can have a different number of neutrons; also an element can gain or lose electrons to become charged, in which case they are chosen ions.
Atomic symbol: The atomic symbol (or chemical element symbol) is an abbreviation chosen to represent an element ("C" for carbon, "H" for hydrogen and "O" for oxygen, etc.). These symbols are used internationally and are sometimes unexpected. For example, the symbol for tungsten is "W" considering another proper noun for that chemical element is wolfram. Also, the atomic symbol for gold is "Au" considering the discussion for golden in Latin is "aurum."
Atomic mass: The standard atomic weight of an chemical element is the average mass of the element written in atomic mass units (amu). Even though each atom has roughly a whole number of atomic mass units, you volition notice that the atomic mass on the periodic tabular array is a decimal; that'due south because the number is a weighted average of the various naturally-occurring isotopes of an element based on their affluence. An isotope is a version of an element with a dissimilar number of neutrons in its nucleus. (To calculate the average number of neutrons in an element, subtract the number of protons (atomic number) from the diminutive mass.)
For example, here'southward how you would calculate the atomic mass of carbon, which has 2 isotopes:
Multiply the abundance of the isotope past its atomic mass:
Carbon-12: 0.9889 10 12.0000 = 11.8668
Carbon-13: 0.0111 x thirteen.0034 = 0.1443
Then, add the results:
xi.8668 + 0.1443 = 12.0111 = diminutive weight of carbon
Atomic mass for elements 93-118: For lab-created trans-uranium elements (elements beyond uranium, which has an diminutive number of 92), there is no "natural" abundance, the Los Alamos National Laboratory (LANL) noted. For these elements, the atomic weight of the longest-lived isotope gets listed on the periodic tabular array, co-ordinate to the International Union of Pure and Applied Chemistry (IUPAC) — the globe dominance on chemical classification and terminology. These atomic weights should be considered provisional, since a new isotope with a longer half-life (how long it takes fifty% of that element to decompose) could exist produced in the future, according to the LANL
The superheavy elements, or those with diminutive numbers in a higher place 104, also fit into this non-natural category. The larger the atom'southward nucleus — which increases with the number of protons inside — the more unstable that element is, generally. As such, these outsized elements are fleeting, lasting mere milliseconds before decaying into lighter elements, according to the IUPAC. For example, superheavy elements 113, 115, 117 and 118 were verified by the IUPAC in December 2015, completing the seventh row, or period, on the table. Several different labs produced the superheavy elements. The atomic numbers, temporary names and official names are:
- 113: ununtrium (Uut), nihonium (Nh)
- 115: ununpentium (Uup), moscovium (Mc)
- 117: ununseptium (Uus), tennessine (Ts)
- 118: ununoctium (Uuo), oganesson (Og)
How is the Periodic Table arranged?
The periodic table is arranged past atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row (called a period) and column (called a group). The tabular array comprises seven rows and eighteen columns. Each element in the same row has the same number of atomic orbitals (the spaces where electrons exist) equally the others in that row or period. That means all of the elements in the 3rd menstruum — sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine and argon — accept 3 atomic orbitals where their electrons reside. Meanwhile, the column or grouping signifies the number of electrons in the atom'due south outermost shell; these are called the valence electrons, and they are the electrons that can chemically bond with valence electrons of other elements. The valence electrons tin can be either shared with another chemical element, a type of covalent bonding, or exchanged in a type of ionic bonding, according to Lumen Learning.
For instance, all of the elements in the second column accept 2 valence electrons; in the third cavalcade, they have three valence electrons. At that place are some exceptions to this rule in the transition elements, which fill the shorter columns at the center of the periodic table. These transition elements
Allow'southward try an case: Nosotros can cull selenium, which has an atomic number of 34, meaning there are 34 total electrons in a neutral atom of selenium. This non-metal resides in Period 4, Grouping 6A. That ways selenium keeps its electrons in four atomic orbitals, and has six valence electrons, or six electrons in its outermost orbital. Yous can likewise figure out how many electrons are in its first, 2d and 3rd orbitals: The offset orbital tin agree a maximum of two electrons, while the 2nd has four suborbitals and then tin hold a total of viii electrons. The third beat out of an cantlet, which consists of 9 suborbitals, can hold a maximum of 18 electrons, according to Florida State Academy'southward Department of Chemical science and Biochemistry. That ways selenium has ii, viii, 18 and 6 electrons in its first, second, third and fourth atomic orbital, respectively.
How is the Periodic Table used today?
By knowing that certain elements that are lumped together on the tabular array have certain characteristics and behaviors, scientists can figure out which ones would be all-time for sure industries and processes. For instance, engineers employ different combinations of elements in Groups 3 and Five of the table to create new semiconductor alloys, such as gallium nitride (GaN) and Indium nitride (InN), according to the National Institute of Standards and Applied science (NIST).
In general, chemists and other scientists can employ the table to predict how certain elements will react with one another. The alkali metals, for example, are in the first cavalcade or group of the table and tend to accept one valence electron so carry a charge of +one. This charge means they "react vigorously with h2o, and combine readily with nonmetals," pharmacist Anne Marie Helmenstine wrote on ThoughtCo. Magnesium, which is in the aforementioned group on the table as calcium, is condign useful as function of alloys for os implants, NIST said. Since these alloys are biodegradable, they serve as a scaffolding and and so disappear later natural bone grows on the structures.
Additional reporting by Traci Pedersen, Live Science contributor
Source: https://www.livescience.com/25300-periodic-table.html#:~:text=California%2C%20San%20Diego.-,Reading%20the%20Periodic%20Table,chemical%20behavior%20of%20the%20element.
0 Response to "What Information About an Atom's Properties Can You Read From the Periodic Table?"
Post a Comment